TRAINING DAYS: 5
(4 days of training + 1 day exam)
CPD CERTIFICATION
31 Credits
EXAM DURATION
3 Hours
EXAM RETAKE POSSIBLE?
Yes. Free one more exam in 12 months
WHAT IS INCLUDED?
Training, PECB exam and certification
Course Overview
ISO 13485 Lead Auditor training enables you to develop the necessary expertise to perform a Medical Devices Quality Management System (MDQMS) audit by applying widely recognized audit principles, procedures, and techniques.
Why Should You Attend?
During this training course, you will acquire the knowledge and skills to plan and carry out internal and external audits in compliance with ISO 19011 and ISO/IEC 17021-1 certification process.
Based on practical exercises, you will be able to master audit techniques and become competent to manage an audit program, audit team, communication with customers, and conflict resolution.
After acquiring the necessary expertise to perform this audit, you can sit for the exam and apply for a “PECB Certified ISO 13485 Lead Auditor” credential. By holding a PECB Lead Auditor Certificate, you will demonstrate that you have the capabilities and competencies to audit organizations based on best practices.
Who Should Attend?
Learning objectives
This training course will help you:
Educational approach
Prerequisites
A fundamental understanding of ISO 13485 and comprehensive knowledge of audit principles.
Course Agenda
Day 1: Introduction to Medical Devices Quality Management Systems (MDQMS) and ISO 13485
Day 2: Audit principles, preparation and launching of an audit
Day 3: On-site audit activities
Day 4: Closing the audit
Day 5: Certification Exam
Examination
The “PECB Certified ISO 13485 Lead Auditor” exam fully meets the requirements of the PECB Examination and Certification Programme (ECP). The exam covers the following competency domains:
Domain 1: Fundamental principles and concepts of a Medical Devices Quality Management System (MDQMS)
Domain 2: Medical Devices Quality Management System (MDQMS)
Domain 3: Fundamental audit concepts and principles
Domain 4: Preparation of an ISO 13485 audit
Domain 5: Conducting an ISO 13485 audit
Domain 6: Closing an ISO 13485 audit
Domain 7: Managing an ISO 13485 audit program
General Information
1. Certification fees are included on the exam price.
2. Training material containing over 450 pages of information and practical examples will be distributed.
3. An attestation of course completion worth 31 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course.
4. In case of exam failure, you can retake the exam within 12 months for free.
Certification
After successfully completing the exam, you can apply for the credentials shown on the table below. You will receive a certificate once you comply with all the requirements related to the selected credential.
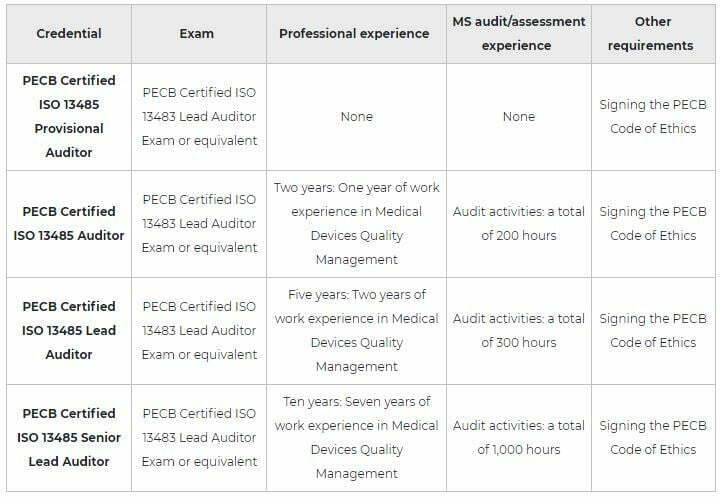
Note: PECB Certified Individuals who do possess the Lead Implementer and Lead Auditor Credentials are qualified for the respective PECB Master Credential, given they have taken 4 additional Foundation Exams which are related to this scheme. For more detailed information about the Foundation Exams and the overall Master Requirements, please go to the following link: https://pecb.com/en/master-credentials.
To be considered valid, these audits should follow best audit practices and include the following activities:
1. Audit planning
2. Audit interview
3. Managing an audit program
4. Drafting audit reports
5. Drafting non-conformity reports
6. Drafting audit working documents
7. Documentation review
8. On-site audit
9. Follow-up on non-conformities
10. Leading an audit team

